Basic things about Organic Chemistry I wish someone had told me before!
Chemistry was initially quite difficult for me to understand. Here are a few basic concepts which when understood perfectly will help. I am assuming you have an idea about some things in chemistry.
Bonds
Chemical bonds are of four basic types:
- Ionic bond
- Covalent bond
- Hydrogen bond
- van der Waals interactions
- Sigma bond
- Pi bond
Pi bond is formed by the lateral overlap of the orbitals. This leads to the formation of double and triple bond. The pi bonds are shorter and stronger than sigma bond.
Nucleophile vs Electrophile
Whenever I saw these two words, I landed up being extensively confused! They sound exactly the same. The solution to this problem came up quite easily, remember that nucleophile means nucleus loving and electrophile is electron loving.
This particular concept is quite important because reaction mechanisms can be easily understood once you get the hang of this.
Reaction mechanism/Synthesis
The key to understanding a reaction mechanism/synthesis is knowing how to read it then proceeding to figure out what exactly happens.
The arrow always shows the movement of electrons. The arrowhead points to the site of less electron density.
Never memorize a synthesis, understand it. Remember the starting material, the reagent and the end product. The steps will automatically come.
The next post covers the rest!




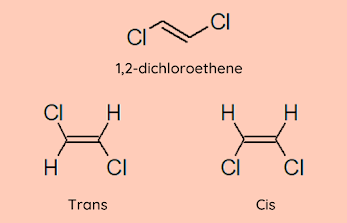
Comments
Post a Comment