Configuration of Geometric Isomers (E/Z and Cis/Trans)
Geometric isomers are formed when the rotation of the Carbon-Carbon bond is restricted either due to a double bond or the carbon-carbon single bond is a part of a chain.
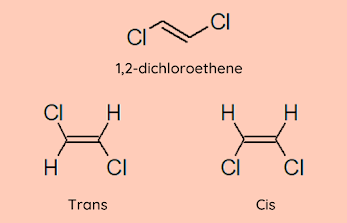
Cis and Trans
This notation is used when both carbons have the same substituent. If the substituents are on the same side, then the isomer is cis else it is trans.
The physical properties of cis and trans isomers vary.
In an acyclic molecule,usually, the stability of a trans isomer is higher than a cis isomer because of steric hindrance between the substituents in the cis isomers.
When the carbons have different substituents, cis/trans nomenclature cannot be used. Here, E/Z is used.
E/Z
First we need to understand how to assign priority to the substituents to denote the E/Z configuration. The priority is denoted by following Cahn Ingold Prelog rules.
- Higher atomic number- higher priority
- In case isotopes are present, higher priority is given to the isotope which possesses higher atomic weight.
- If the atoms are identical, the first point of difference is found and the chains are compared.
- Multiple bonds are considered multiples of the same atom.
In E/Z nomenclature,
E stands for Entgegen which, in German, means opposite.When groups of higher priority are on the opposite sides of the double bond the bond is assigned the configuration E.
Z stands for Zusammen which, in German, means together.When groups of higher priority are on the same side of the double bond the bond is assigned the configuration Z.
For the alkene carbon on the left, Br gets a higher priority over Cl due to its higher atomic number.
For the alkene carbon on the right, C gets a higher priority over H due to the atomic number.
In the first compound, the higher priority groups are on the same side, so, Z configuration. Whereas, in the second compound, higher priority groups are on the opposite , so, E configuration.




Comments
Post a Comment